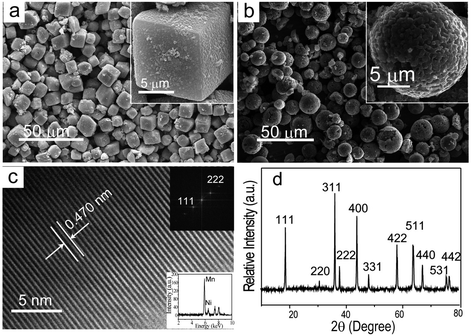
Lithium-ion batteries are being actively pursued for transportation and stationary storage of renewable energies. The success of lithium-ion technology for these large-cell applications depends critically on the development of new electrode materials that can offer high energy and power at an affordable cost and acceptable safety. In this regard, the LiMn1.5Ni0.5O4 (LMNO) spinel has become appealing as it offers a high operating voltage of 4.7 V with high intrinsic rate capability due to the facile 3-dimensional lithium-ion diffusion in the spinel lattice. Despite the intense interest and continuous investigation, morphologically-controlled synthesis of LMNO remains a challenge because of the high synthesis temperatures needed to form the spinel phase with acceptable performance. Conventional synthesis methods, including those based on sol–gel formation or solid state reaction, usually produce irregular particles with low tap density and consequently low volumetric energy density, a parameter critical for transportation applications especially considering that only limited space will be available for batteries in vehicles. Therefore, controlled synthesis of cathode materials with favorable shapes and sizes that can maximize the volumetric energy density for practical applications is critical to realize the full potential of these next generation cathode materials.

