An Advanced Selenium-Carbon Cathode for Rechargeable Lithium-Selenium Batteries
Chun-Peng Yang, Sen Xin, Ya-Xia Yin, Huan Ye, Juan Zhang, Yu-Guo Guo*
Angew. Chem. Int. Ed., 2013, DOI: 10.1002/anie.201303147, in press
We report a Se composite cathode material, in which Se is confined as cyclo-Se8 molecules in the mesopores of an ordered mesoporous carbon (CMK-3) matrix. When assembled into Li-Se batteries with a water-soluble binder (sodium alginate, SA), the Se/CMK-3 composite exhibits a novel electrochemical behavior with a single plateau in the discharge/charge process. Ex situ Raman and X-ray diffraction (XRD) data suggest that this behavior is due to the evolution of cyclo-Se8 molecules into chain-like Sex molecules in the carbon channels. Given the high electrochemical activity of the chain-like Sex molecules and the strong interaction between them and carbon mesopores, the as-prepared Se cathode shows a high capacity approaching the theoretical value of Se and exhibits favorable capacity retention upon cycling.
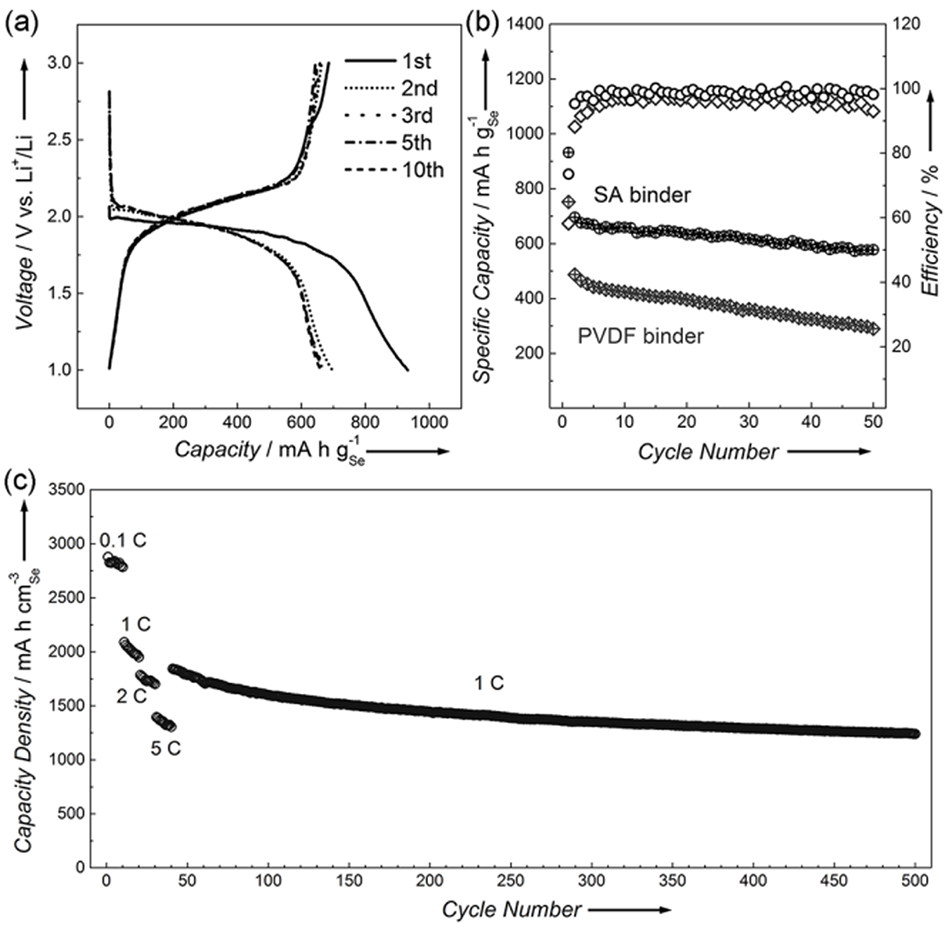
Figure 1. (a) Glavanostatic discharge/charge voltage profiles of Se/ CMK-3 with SA binder in the voltage range of 1.0-3.0 V (vs. Li+/Li) at 0.1C. (b) Cycling performances and Coulombic efficiencies of Se/CMK-3 with SA and PVDF binders (rate: 0.1 C). (c) Reversible capability density of Se/CMK-3 at 0.1 C, 1 C, 2 C and 5 C, and long cycling performance at 1 C.
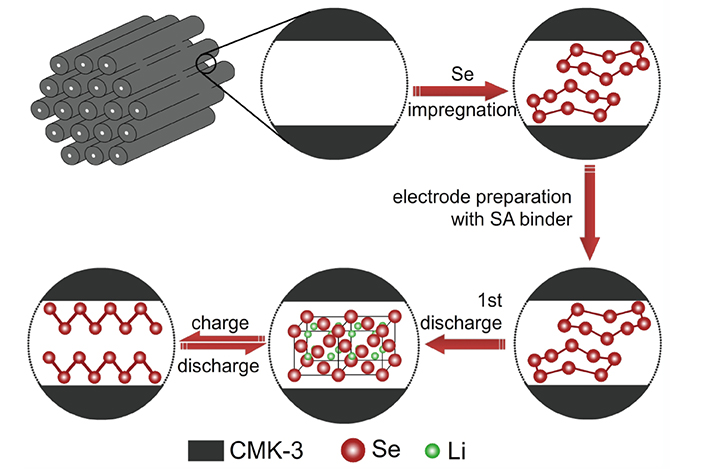
Figure 2. Schematic illustration showing the lithiation/delithiation processes of Se/CMK-3.

