Author:lidan
There is no doubt about the significance of Li-based batteries for our future lives, yet there is also no doubt about the necessity of finding better electrode materials. A key problem in Li-battery research is guaranteeing sufficiently rapid transport of both ions and electrons.
Under the supports of the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Chinese Academy of Sciences, and Institute of Chemistry, researchers of the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology performed a series of studies on the development of high-performance electrode materials for lithium-ion batteries. (Adv. Mater., 2008, 20, 2878; Adv. Mater., 2008, 20, 1160; Adv. Mater., 2009, 21, 2710; Adv. Mater., 2010, 22, 4591; Adv. Mater., 2011, 23, 4415).
Recently, together with German researchers they have demonstrated that very effective synergism could be introduced by using two-phase structures such as the coaxial nanocables (Fig. 1a). They can be used for designing superior electrode materials with improved performance in terms of power (rate), energy, and cycling behavior. The cable morphology also allows for a dense packing of electroactive materials. In addition to the bifunctionality on the nanoscale, it can easily form a micrometer-scale mixed-conducting network with carbon black as well (Fig. 1b). In the specific case of CNT@TiO2 core/porous-sheath coaxial nanocable, on one hand, the benefit of CNT for TiO2 storage consists in the electronic wiring principle (i.e., the CNT core providing sufficient e- for the TiO2 sheath). On the other hand, the benefit of nanoporous TiO2 for CNT is the almost unperturbed Li+ supply for the CNT core, most probably because of the porosity and the small thickness of the passivation layer. It is the synergism of the two parts that leads to a high, fast and stable lithium storage material. This work has been published in Chem. Mater. (2010, 22, 1908–1914) and has been highlighted by the RSC Chemistry Word (March 2010, P26).
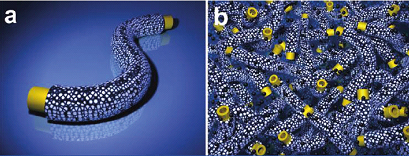
Figure 1. Symbiotic coaxial nanocables. (a) Schematic illustration of symbiotic coaxial nanocables with electronically conducting core (e.g., CNTs) and Li+providing nanoporous sheath (e.g., TiO2). (b) Corresponding schematic illustration of the effectively mixed conducting 3D networks formed by the nanocables and carbon black. (Image by Yu-Guo Guo et al.)
They have also extended the concept to Cu-nanowire-containing nanocables and demonstrated with Cu@TiO2 nanocables. Benefiting from the remarkable size effect of tiny TiO2 crystallites and integrated Cu nanowires as mechanical supports and efficient current collectors, the as-prepared Cu@TiO2 nanocable-like electrode material with the smaller TiO2 grain size shows an outstanding high rate performance when it is used as an electrode material in lithium batteries. (Phys. Chem. Chem. Phys., 2011, 13, 2014–2020).
More recently, they have also developed a concept of using nanocables directly grown from the current collector for the electrodes of LIBs and realized by fabricating Cu-Si and Cu-Si-Al2O3 nanocable arrays (Fig. 2). The conductive Cu cores that are anchored to the copper foil act as both current collectors and structural reinforcements for the Si shell of the nanocables. The outer surface of the nanocables is readily modified by an additional sheath of Al2O3, which provides a stable Si/electrolyte interface and triggers stable SEI formation. Consequently, both nanocables show excellent electrochemical performance including high specific capacity and cycling stability. After coating with an Al2O3 sheath, the Cu-Si-Al2O3 nanocables show a remarkable high rate capability.(Adv. Mater., 2011, 23, 4415)
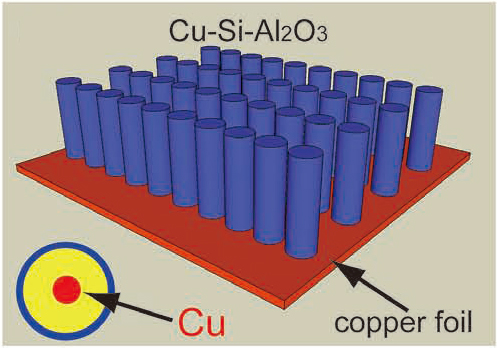
Figure 2. Schematic illustration of the Cu-Si-Al2O3 nanocable array. (Image by Yu-Guo Guo et al.)
They were invited to write a PERSPECTIVE article for the high-impact RSC journal of Energy & Environmental Science. In the published PERSPECTIVE articleentitled “Better lithium-ion batteries with nanocable-like electrode materials”, they give an overview of the design, synthesis, and applications of nanocable-like structures in lithium-ion batteries and highlight some of the latest achievements in this area. It is exciting that the future of lithium-ion batteries is quite bright in view of the high specific capacity, much improved rate performance, as well as superior cycling stability brought by the nanocable-like electrode materials. Furthermore, the PERSPECTIVE was selected as the “Back Cover” (Fig. 3). (Energy Environ. Sci., 2011, 4, 1634-1642)

Figure 3. Back Cover of Energy. Environ. Sci., Issue 4, 2011. (Image by Yu-Guo Guo et al.)

