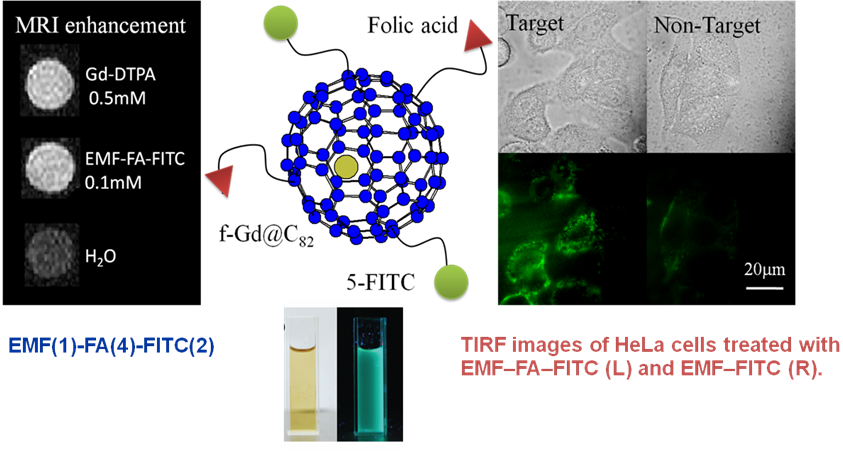
Gd@C82(OH)22, a remarkable gadofullerenol, has been found to be a competitive antitumor candidate with low cytotoxicity and decreases the activities of enzymes related to reactive oxygen species (ROS) generation in vivo. Therefore, strategic design of gadofullerene derivatives is possible to integrate multifunctionality into one entity. we report an easy method to fabricate amino groups bearing gadofulleride for magnetic resonance imaging/fluorescent (MRI/FL) integrative imaging. To the best of our knowledge, this is the first example of an amine terminal endohedral fulleride produced by a one-step reaction. Besides the highly efficient MRI performance, the as-synthesized gadofulleride exhibits multi-wavelength excited imaging properties, which make it a promising candidate for dual modality imaging along with biolabeled carriers. Moreover, the highly efficient scavenging capability of the gadofulleride toward hydroxyl radicals was found, suggesting the concomitantly potential application for combating the ROS induced cellular impairment. (Nanoscale, 2012, 4 (12), 3669)