2D chirality of molecular assembly on solid surfaces has gained wide spread interest for its fundamental significance and potential applications in enantioselective heterogeneous catalysis, chiral separation, chiroptical materials and sensors. A variety of chiral molecular assemblies have been acquired on solid surfaces from chiral or achiral molecules. However, the origin, transfer, and amplification of chirality on 2D remain unclear. The spontaneous resolution of the handedness of the assemblies shows no bias and typically leads to globally racemic surfaces.
Under the supports of the Chinese Ministry of Science and Technology, the National Natural Science Foundation of China, and the Chinese Academy of Sciences (CAS), Prof. Wan Lijun and Prof. Wang Dong’s groups from the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, CAS, have long-term commitment on study of surface chirality and have made series progress. (J. Am. Chem. Soc., 2008, 130, 8878-8879; J. Am. Chem. Soc., 2010, 132, 5598-5599; 2011, 133, 21010-21015) Recently, they made great progress on the globally homochirality control of 2D molecular assembly by using co-absorbers. The results have been published in Nature Communications (2013, 4:1389). http://www.nature.com/ncomms/journal/v4/n1/full/ncomms2403.html
Researchers devised a multiple hydrogen bonding stabilized chiral networks by co-assembly of 5-(benzyloxy)-isophthalic acid derivative (BIC) and 1-octanol on highly oriented pyrolytic graphite. By controlling the handedness of the carbon atom adjacent to the hydroxyl group in octanol, globally homochiral network assembly with preferred handedness is succefully achieved. The stereoselective interactions between BIC aggregation aggregations and octanol molecules play important roles in the induction of the homochirality. The chirality of the chiral carbon in octanol is transferred to the BIC/octanol unit via the stereoselective interactions and further hierarchically amplified to the 2D assembly (Figure 1). Moreover, nonlinear amplification of the globally homochirality to the enantiomeric excess of the chiral octanol was observed. When the excess of one enantiomer of chiral octanol reaches ≥ 5.2%, global homochirality of the assembled networks can be realized (Figure 2). It is the first time to demonstrate the nonlinear chirality amplification in 2D molecular assemblies from achiral molecules. The global homochirality can be memorized by site replacement of chiral octanol with other achiral co-absorbers.
Such an induction and nonlinear chirality amplification effect promises a new approach towards 2D homochirality control and may reveal important insights into asymmetric heterogeneous catalysis, chiral separation and chiral crystallization.
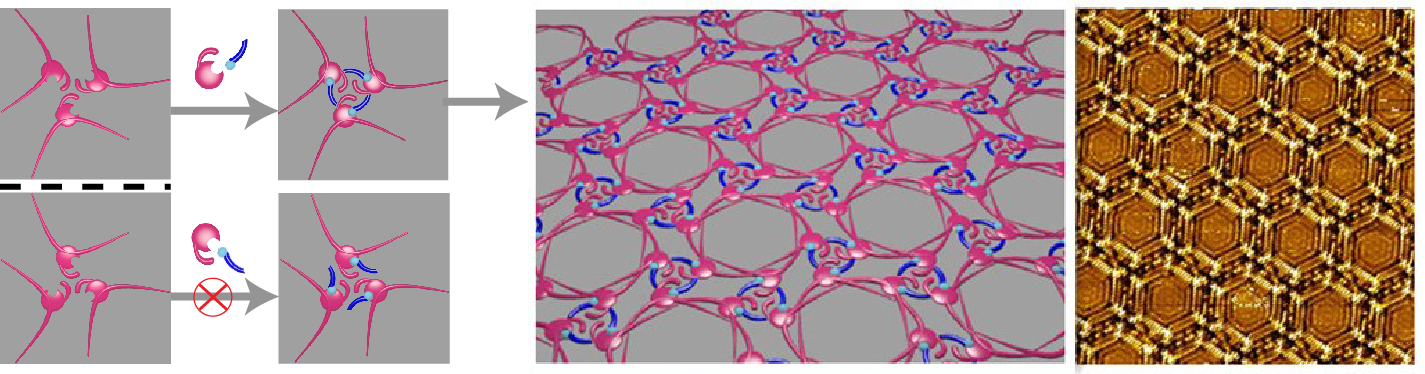
Figure 1. Illustration of the chirality control via the stereoselective interactions between BIC aggregations and chiral octanol molecules.
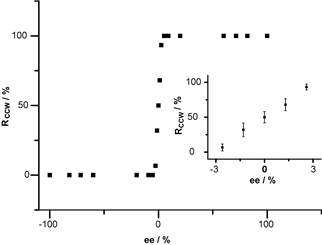
Figure 2. Nonlinear amplification of the CCW networks to the enantiomeric excess of the (S)-2-methyl octanol in the solution.

