To meet the rapid developments in various application fields such as consumer electronics, electric vehicle and energy-storage power source, there is an urgent need to further improve the properties of lithium-ion batteries in terms of energy densities, power densities, cycle life and safety. One of the crucial issues to achieve this goal lies in the development of advanced high-performance electrode materials.
Rencently, Prof. WAN Lijun and Prof. GUO Yuguo’s groups from the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences (ICCAS), made series progress on the development of high-performance electrode materials for lithium-ion batteries. They took advantage of the idea of “Nanocarbon Networks” to carry out rational structural designs for advanced electrode materials, which greatly improved the electrochemical performances of various kinds of nanostructured cathode and anode materials (J. Am. Chem. Soc., 2012, 134, 2512; Energy. Environ. Sci., 2012, 5, 5221; Adv. Energy Mater., 2012, 2, 1086; Chem. Commun., 2012, 48, 2198; Chem. Commun., 2012, 48, 10663; J. Mater. Chem., 2012, 22, 17456; ACS Appl. Mater. Interfaces, 2012, 4, 2824; ACS Appl. Mater. Interfaces, 2012, 4, 4858; Phys. Chem. Chem. Phys., 2012, 14, 2934). On the invitation of the editor of Accounts of Chemical Research, they have published a review article titled “Nanocarbon Networks for Advanced Rechargeable Lithium Batteries”. In the Account paper, they summarized recent progress in the structural design, chemical synthesis, and characterization of the electrochemical properties of nanocarbon networks for Li-ion batteries and the next generation rechargeable lithium batteries, such as Li-S and Li-O2 batteries. In addition, they also addressed the ways in which nanocarbon networks can expand the applications of rechargeable lithium batteries into the emerging fields of stationary energy storage and transportation (Acc. Chem. Res., 2012, 45, 1759).
In recent years, Prof. WAN Lijun and Prof. GUO Yuguo’s groups have focused on the design, fabrication and properties of advanced electrode materials for Li-ion batteries (Adv.Mater., 2008, 20, 2878;Adv. Mater., 2008, 20, 1160;Adv. Mater., 2009, 21, 2710;Adv.Mater.,2010, 22, 4591; Adv. Mater.,2011, 23, 4415;Energy. Environ. Sci., 2011, 4, 1634). Through systematic studies, they found that the nanoporous three-dimensional conducting networks, which are formed by various nanocarbon building blocks (carbon nanoparticle, carbon nanotube, graphene and nanoporous carbon), can effectively disperse the nanoparticles of the active electrode materials to prevent them from agglomerating, provide rapid pathways for Li ions and electrons to reach the surface of each active nanoparticle, and hence make full use of the potential kinetic advantages of the nanostructured electrode materials (Fig. 1). In this way, they developed many electrode materials with high specific capacities and favorable high-rate performances for Li-ion batteries.
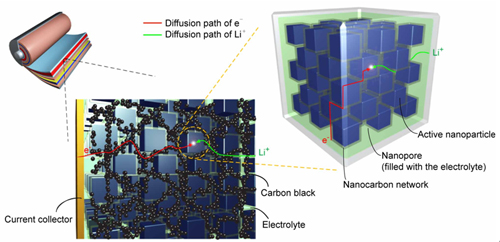
Figure 1. Schematic illustration of the hierarchically mixed conducting network of electrode with nanocarbon networks in a lithium-ion battery. (Image by GUO Yuguo)
With this concept, the researchers made series progress in using graphene to build the three-dimensional conducting networks in hybrid cathode and anode materials, and developing many efficient assembly methods for such structures. (1) They found that, with 1-Methyl-2-pyrrolidinone (NMP) as the dispersant, a uniform combination of graphene with LiNi1/3Mn1/3Co1/3O2 (LMNC, a layered cathode material) nanoparticles or organic radical polymer (PTMA) cathode material can be achieved (Fig. 2a). Significantly improved kinetic performances have been observed in these cathode materials (Phys. Chem. Chem. Phys.,2012, 14, 2934; Energy. Environ. Sci., 2012, 5, 5221). (2) For the high-capacity alloy anode materials, they developed a preparation method, which combines the freeze-drying method and the thermal reduction method to well insert silicon nanoparticles in graphene nanosheets (Chem. Commun., 2012, 48, 2198). They also took advantages of the negatively-charged surfaces of Si nanoparticles (with SiOx surface) and graphene oxide, and developed an electrostatic layer-by-layer assembly technique by using positively-charged polymer electrolyte as the medium to prepare a Si/graphene nanocomposite anode material (Fig. 2c). With this method, an efficient graphene coating on the Si nanoparticles can be achieved, which substantially improves its cycling and high-rate performances (Adv. Energy Mater.,2012, 2, 1086). (3) Recently, they have proposed a double-protection design concept for high-capacity alloy anode material (Fig. 2b). To be specific, they used a core-shell structured nanocarbon shell in combination with the three-dimensional graphene network to solve the problems concerning the high-capacity alloy anode material (i.e., dramatic volume expansion, and surface/interface and kinetic problems). For example, they developed a Ge@C/graphene nanocomposite anode material with high specific capacity, long cycle life and excellent high-rate performance (J. Am. Chem. Soc., 2012, 134, 2512).
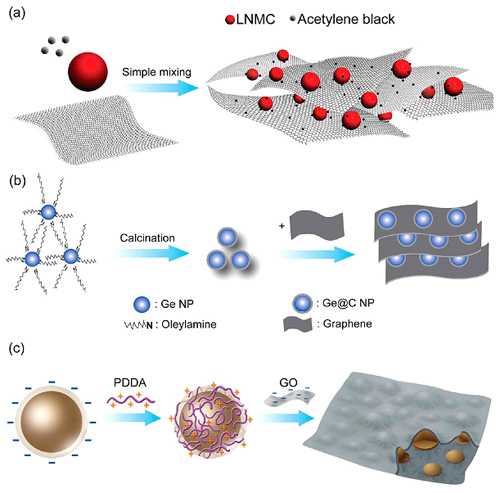
Figure 2. Preparation of hybrid electrode materials with three-dimensional graphene networks: (a) simple mixing method; (b) “double-protection” method; (c) layer-by-layer assembly method. (Image by GUO Yuguo)

